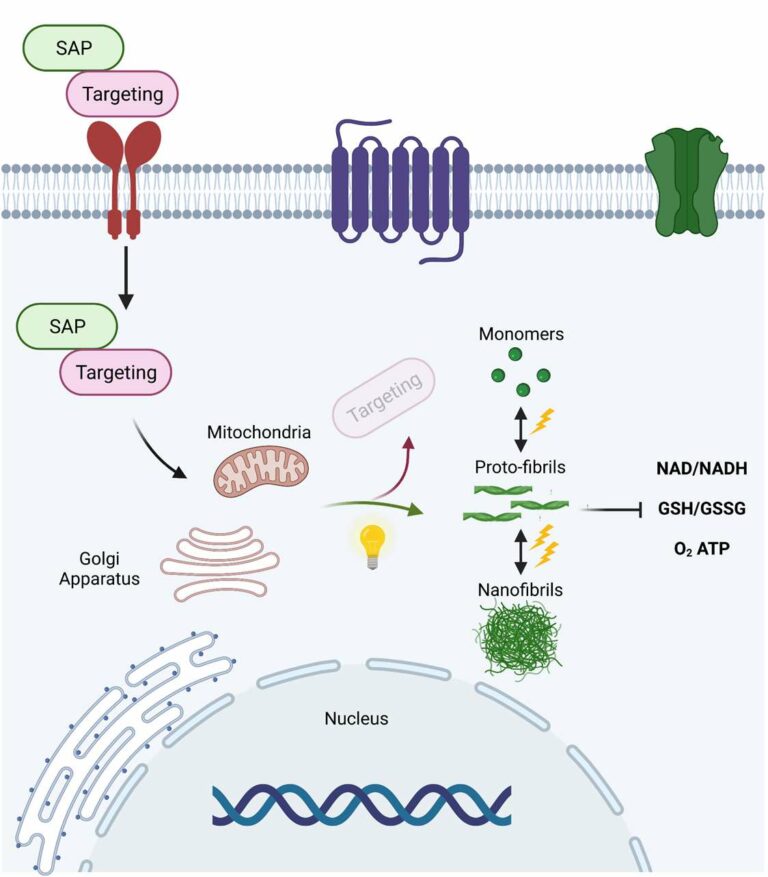
Protein aggregation into fibres inside cells is an ever-present process associated with human disease, but its molecular underpinnings and impact on cellular function are poorly understood. common approach to addressing that is to study how molecular malfunction leads to protein aggregation. The proposed project follows the opposite logic, and will generate and characterize light-inducible, phase-separated polypeptide aggregates (optoaggregates) that assemble in different subcellular compartments of living cells. These phase-separated nanostructures will affect biological functions purely through structure formation with exceptional spatiotemporal control. Non-assembling short peptide precursors will be modified with bio-recognition units that allow cellular uptake and organelle targeting. After irradiation with light, the peptide precursors undergo different stages of self-assembly. The peptide scaffold determines the kinetics of supramolecular polymerization, the extent of phase separation, the morphology of the formed nanostructures as well as their rheological properties and stability. Correspondingly, irradiation with light offers spatiotemporal control over the ratio of monomers, oligomers, proto- and mature nanostructures by gating the amount of active assembling peptides at any given time. These proportions of formed phase-separated nanostructures will subsequently be mapped to their impact on cellular functions, i.e., viability, proliferation, redox and calcium dynamics, metabolism and stress responses. By offering the possibility to control how structure formation proceeds in cells, we can provide a versatile toolbox for studying each aggregation stage in the desired cell type, which is currently unachievable by biological means. Ultimately, this contribution will fill large gaps in our understanding of how protein aggregation and cellular malfunction affect each other. Our findings will represent new insights into the mechanism and progression of protein aggregation diseases to raise the prospects of therapies.