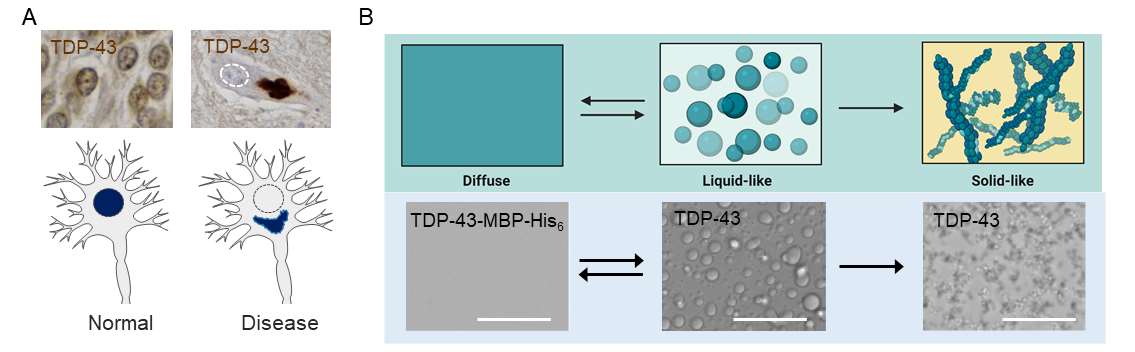
Post-translational modifications (PTMs) play an important role in modifying protein function and cell physiology, but are also linked to many diseases, including neurodegenerative disorders. Several proteins that form insoluble aggregates in neurodegenerative patients, for example, tau in Alzheimer’s disease, and TAR DNA-binding protein of 43 kDa (TDP-43) or fused in sarcoma (FUS) in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), are highly post-translationally modified. However, it is largely unclear how these PTMs alter a proteins’ molecular and polymer behaviour or their functional properties in cells, nor is it known which PTMs are specific to physiological conditions and which are associated with pathology.
Using a combination of biochemical and cellular studies (Dormann) and multi-scale simulations (Stelzl), we aim to understand the roles of PTMs on the neurodegeneration-linked RNA-binding proteins TDP-43 and FUS. In the first funding period, we will focus on known disease-associated PTMs, in particular hyperphosphorylation of TDP-43 and arginine methylation in FUS. We will investigate the phase-separation (PS)/aggregation behaviour of TDP-43 species carrying defined phosphomimetic mutations or phosphosites in different regions, and will study the interactions of differentially modified TDP-43 species with negatively charged biopolymers [RNA, ssDNA, poly(ADPribose) (PAR)], as well as their localization and RNA-processing functions in cells. Moreover, we will investigate how arginine methylation of FUS-RGG/RG regions affects the structural and physical properties of FUS condensates, including mesoscale cluster formation, coacervation with RNA, ssDNA and PAR), as well as the RNA-binding specificity of FUS. Finally, we will seek to establish a pipeline to identify physiological and stress/mislocalization-induced PTMs on TDP-43 and FUS using proteomics, starting with phospho-proteomics in this funding period. In the long term, this approach will enable us to identify physiological and disease-linked PTMs of TDP-43 and FUS, and to understand their impact on the proteins’ PS/aggregation behaviour, on their interactions with other biopolymers, and on their cellular localization and physiological functions. We will add to the associative-polymers paradigm of protein phase behaviour, study homotypic and heterotypic interactions in self-assembly, and elucidate the molecular driving forces of coacervation of proteins with nucleic acids.
Related publications:
- L. A. Da Gruijs Silva, F. Simonetti, S. Hutten, …, V. Dötsch, D. Edbauer, G. Hummer, L. S. Stelzl, D. Dormann*, Disease-linked TDP-43 hyperphosphorylation suppresses TDP-43 condensation and aggregation. EMBO J., e108443 (2022).
- E.L. Sternburg, L.A. Gruijs da Silva, D. Dormann*, Post-translational modifications on RNA-binding proteins: accelerators, brakes, or passengers in neurodegeneration? Trends Biochem. Sci. 47, 6 (2022).
- M. Hofweber#, S. Hutten#, …, T. Madl, D. Dormann*, Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 173, 706 (2018).
- L. M. Pietrek#, L. S. Stelzl#, G. Hummer, Hierarchical Ensembles of Intrinsically Disordered Proteins at Atomic Resolution in Molecular Dynamics Simulations, J. Chem. Theory Comput. 16, 725 (2020).
- Z. Benayad, S. von Bülow, L. S. Stelzl, G. Hummer, Simulation of FUS Protein Condensates with an Adapted Coarse-Grained Model, J. Chem. Theory Comput. 17, 525 (2021).
- L. S. Stelzl*, L. M. Pietrek*, …, B. Schuler, M. Zweckstetter, G. Hummer, Global Structure of the Intrinsically Disordered Protein Tau Emerges from its Local Structure, JACS Au. 2(3), 673–686 (2022)


